Stem Cells in Organ Regeneration
Every day our body makes and loses billions of cells. This continuous regeneration is sustained by stem cells, which act as a lifelong reservoir by self-renewing and differentiating into the different lineages of a tissue.
In the Greco lab, our goal is to define how tissues maintain themselves throughout the course of our lives in the face of continuous cellular turnover, frequent injuries, and spontaneous mutations.
Principles of Tissue Dynamics and Function Captured by Imaging | Dr. Valentina Greco
Despite the highly dynamic nature of this process, the field has been limited by the inability to track the same cells over time and interrogate their behaviors in a live mammal. To overcome this challenge, we took inspiration from the developmental biology field to develop novel tools that integrate imaging of stem cells in their niche in live mice with genetics and cell biological approaches. This allows us to understand the complex orchestration of tissue regeneration using the skin as model system.
We have leveraged our non-invasive imaging approach together with mathematical modeling, transcriptomics, metabolomics and machine learning - and made discoveries that impact well beyond the regeneration field, into cancer, metabolism and developmental biology. Some of these discoveries have been that
Stem cells regulate epithelial regeneration by coupling their behaviour to their neighbors. By tracking and manipulating proliferation and differentiation we have discovered that this local control is critical for both regeneration, structure and function of the epidermis.
Stem cells defend against tumor formation. By using oncogenic mosaic models of wildtype and Hras cells we discovered that healthy stem cells increase their proliferation to suppress oncogenesis via selective utilization of EGFR and p21 pathways.
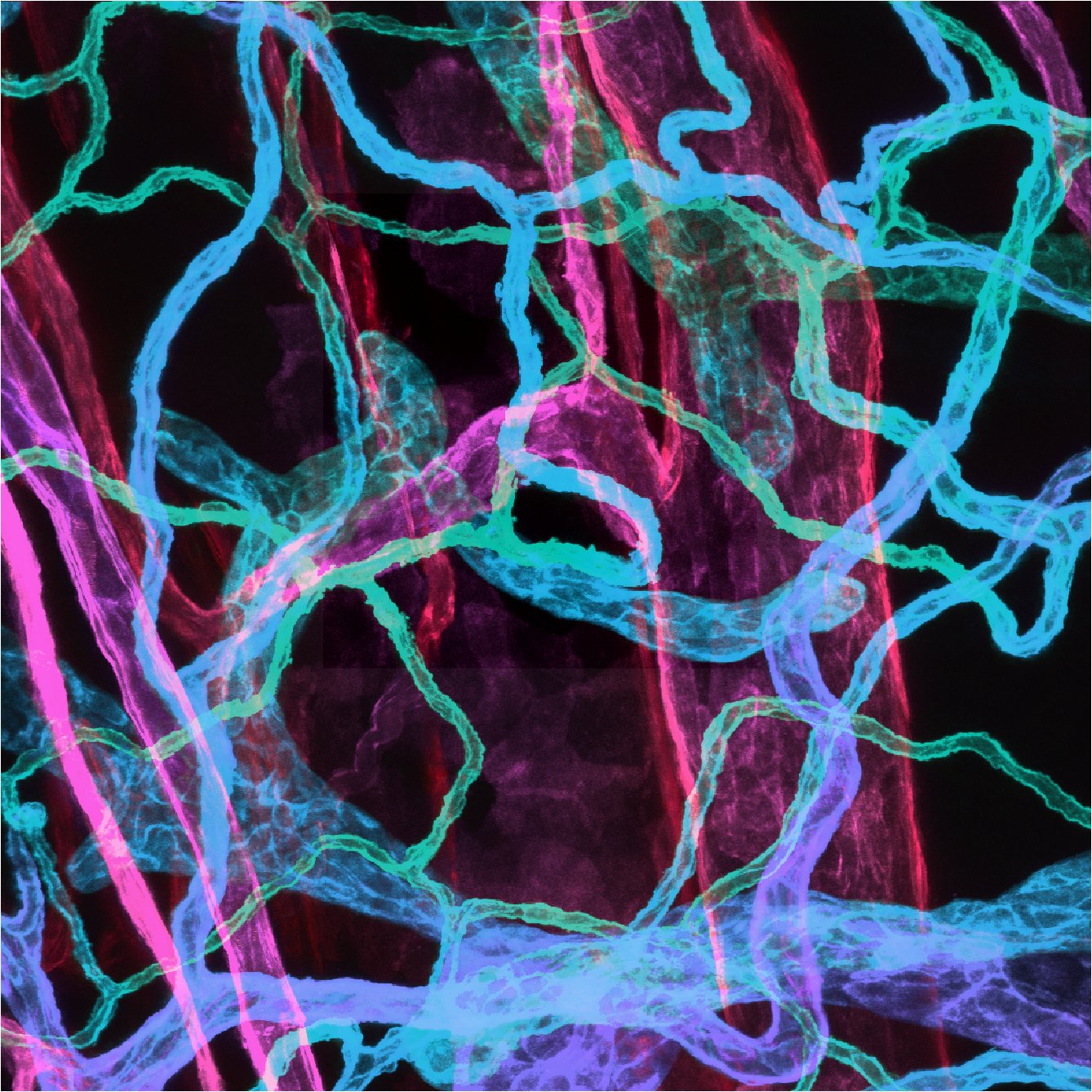
Niche cells such as fibroblasts and endothelial cells have distinct roles depending on age. For example we discovered an inverse functional effect on epithelial regeneration in neonate stages versus adult stages.
These breakthroughs advance our long-term goal of understanding neighborhood regulation, epithelial tolerance and niche interaction. On-going projects are looking at understanding the mechanism of skin tolerance to mutations as well as how the different tissue types such as epithelium, fibroblasts, and vasculature contribute to skin homeostasis throughout our lifetimes.