Oncogenic Tolerance
Deep sequencing of aged yet phenotypically normal human skin, blood, and esophagus has revealed that tissues are a mosaic of mutated cells. Yet it remains unclear how normal tissues cope with the presence of mutant cells, and what goes awry during cancer.
Using our powerful live imaging approach, we demonstrated that inducing the activating mutation of a critical regulator of growth, Wnt/β-catenin, heterogeneous tumors made up of both mutant β-catenin cells and wild-type cells formed, revealing a dynamic interaction between mutant and wild-type cells (Deschene* & Myung*, 2014). Surprisingly, the wild-type cells within these growths induced their ultimate regression (Brown* & Pineda*, 2017), suggesting that wild-type cells in our tissues may battle emerging cancerous growths.
Cells carrying Wnt/b-catenin mutations can drive aberrant growths. Surprisingly, these growths are eliminated from the tissue and do not re-emerge over time as shown here in murine hair follicles.
Our lab and others have previously demonstrated that broad, epidermal activation of mutant Hras results in the development of benign skin tumors. However, we found that when Hras is expressed in only a fraction of epithelial stem cells, mutant cells increase their relative proliferation to outcompete wild-type neighbors, yet are integrated into clinically normal structures that remain under homeostatic control (Pineda 2019).
The Hras mutant hair follicle has more mitotic events (numbered arrows) than the wild-type control.
Surprisingly, we recently discovered that the injury-repair process switches the competitive balance between Hras mutant and wild-type epithelial stem cells: wild-type cells selectively increase their proliferation, thereby suppressing the expansion of Hras mutant cells and preventing tumorigenesis (Gallini 2023). Mechanistically, this switch is due to differential responses to injury-induced MAPK signaling. This opens the door to potential targeted therapeutics harnessing the power of our tissues to preserve overall architecture and homeostasis even when neighbors harbor oncogenic mutations.
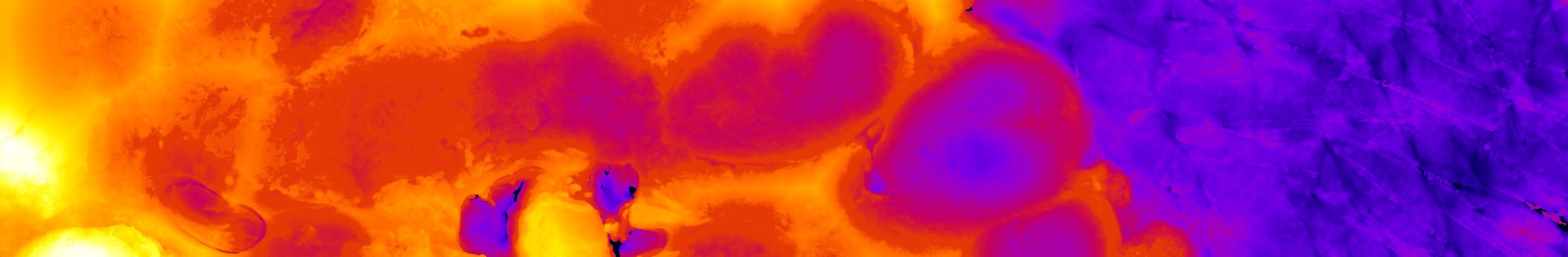
ERK signaling dynamics in hair follicle stem cells.
To understand the earliest molecular responses to the emergence of oncogenic cells in the skin, we have been adapting our live imaging approach to visualize fast-scale molecular states and events at a single-cell level before and after introducing oncogenic mutant cells. Recently, by tracking the metabolites NADH and FAD, we discovered that introducing either β-catenin mutant cells or Hras mutant cells leads to a rapid change in metabolic state of wild-type neighbors, but the “winner” cells in each model recover their metabolic state (Hemalatha 2022).
We also recently captured real-time ERK signaling in hair follicle stem cells and found that introduction of oncogenic Kras changes ERK signaling dynamics to cause tissue deformation (Xin 2022). These advances to our imaging approach paired with orthogonal approaches such as metabolomics, transcriptomics, and pharmacological perturbations are giving us a window into the earliest molecular responses to oncogenesis.
Moving forward, we are determined to understand the combination of factors that allow a tissue to integrate, tolerate, and/or eliminate cells carrying different mutations while maintaining a homeostatic steady state. We also aim to capture the critical cellular behaviors and molecular cross-talk occurring within and between mutant and wild-type populations that drive cancer emergence.



